Multiple Choice
The number of moles of MgO produced when 0.20 mole of 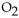 reacts completely is
reacts completely is
A) 0.80 mole.
B) 0.10 mole.
C) 0.20 mole.
D) 0.40 mole.
E) 0.60 mole.
Correct Answer:

Verified
Correct Answer:
Verified
Q57: For the question(s)that follow, consider the following
Q58: What is the molar mass of <img
Q59: Barium chloride and sodium sulfate react according
Q60: Any reaction that absorbs 150 kcal of
Q61: The following reaction takes place when an
Q63: The molar mass of potassium is<br>A) <img
Q64: The molar mass of calcium hydroxide, <img
Q65: The molar mass of C<sub>3</sub>H<sub>8</sub>O<sub>2</sub> is<br>A)52.0 g.<br>B)69.0
Q66: What type of reaction is: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q67: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8494/.jpg" alt=" A)125