Multiple Choice
For the question(s) that follow, consider the following equation. 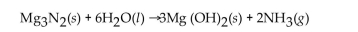
-What is the correct form of the conversion factor needed to convert the number of moles of 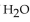 to the number of moles of
to the number of moles of 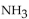 produced?
produced?
A) 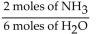
B) 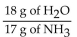
C) 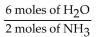
D) 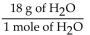
E) 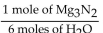
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q52: What type of reaction is the following?
Q53: Acetylene gas, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Acetylene gas,
Q54: How many moles are in 37.6
Q55: When 4 moles of aluminum are allowed
Q56: What is the coefficient of hydrogen, <img
Q58: What is the molar mass of <img
Q59: Barium chloride and sodium sulfate react according
Q60: Any reaction that absorbs 150 kcal of
Q61: The following reaction takes place when an
Q62: The number of moles of MgO produced