Multiple Choice
Barium chloride and sodium sulfate react according to the following equation. 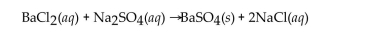 Answer the question(s) that follow about this reaction.
Answer the question(s) that follow about this reaction.
-How many grams of barium sulfate can be produced from 20.8 g of barium chloride?
A) 23.3 g
B) 233 g
C) 137 g
D) 1.37 g
E) 2.33 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q54: How many moles are in 37.6
Q55: When 4 moles of aluminum are allowed
Q56: What is the coefficient of hydrogen, <img
Q57: For the question(s)that follow, consider the following
Q58: What is the molar mass of <img
Q60: Any reaction that absorbs 150 kcal of
Q61: The following reaction takes place when an
Q62: The number of moles of MgO produced
Q63: The molar mass of potassium is<br>A) <img
Q64: The molar mass of calcium hydroxide, <img