Multiple Choice
When 4 moles of aluminum are allowed to react with an excess of chlorine gas, 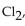 how many moles of aluminum chloride are produced?
how many moles of aluminum chloride are produced?
A) 1 mole
B) 2 moles
C) 3 moles
D) 4 moles
E) 5 moles
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: For the question(s)that follow, consider the following
Q51: In this reaction, what is the substance
Q52: What type of reaction is the following?
Q53: Acetylene gas, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Acetylene gas,
Q54: How many moles are in 37.6
Q56: What is the coefficient of hydrogen, <img
Q57: For the question(s)that follow, consider the following
Q58: What is the molar mass of <img
Q59: Barium chloride and sodium sulfate react according
Q60: Any reaction that absorbs 150 kcal of