Multiple Choice
Exhibit 2A The structure of ATP with various groups labeled. Group III is the entire phosphate group. 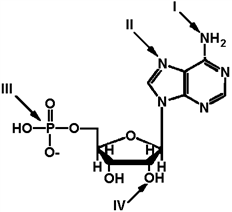
Refer to Exhibit 2A. Which of the groups could not act as a proton acceptor in a hydrogen bond?
A) I
B) II
C) III
D) IV
E) All can accept a hydrogen in a hydrogen bond.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Hydrogen bonds<br>A) play an important role in
Q3: What is the pH of a solution
Q4: The water molecule is polar because:<br>A) Electrons
Q5: Exhibit 2B Contains information on the pK's
Q6: If a solution has a pH =
Q7: Distinguish between the hydrogen bonding found
Q8: Which of the following is true regarding
Q9: Which of the following acids would serve
Q10: Exhibit 2B Contains information on the pK's
Q11: Which of the following is true?<br>A) The