Multiple Choice
Exhibit 8-1 The following question(s) relate to the diagram below: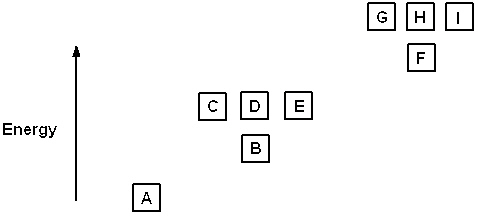
Refer to Exhibit 8-1. If boxes A, B, C, and D each are filled with two electrons and box E has one, the neutral atom that would have this arrangement of electrons is:
A) Ne
B) N
C) O
D) F
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q2: The answer which correctly arranges the species
Q3: What ion has the ground state electron
Q4: Three statements are listed below. Pick the
Q5: How many valence electrons are present in
Q6: Which element has the valence electrons 3s<sup>2</sup>3p<sup>2</sup>?<br>A)
Q7: The valence shell orbital configuration of the
Q8: How many unpaired electrons does the ground
Q9: What is the order for increasing atomic
Q10: The ground state electron configuration 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>2</sup> would
Q11: Which species is isoelectronic with As?<br>A) Ga<sup>2+</sup><br>B)