Multiple Choice
Sodium chloride, an ionic compound, should be most soluble in which solvent? ( = dielectric constant)
(a) hexane
(b) 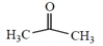
acetone
(c) 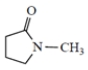
-methylpyrrolidone
(d) 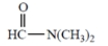
(e) 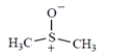
dimethylsulfoxide
A) compound a
B) compound b
C) compound c
D) compound d
E) compound e
Correct Answer:

Verified
Correct Answer:
Verified
Q1: When two hydrocarbon molecules (such as hexane),
Q2: This is the predominant form of the
Q3: The boiling point of pentane is 36
Q4: In each case, identify the better solvent
Q5: In which solvent should NaCl (an ionic
Q7: Dissolving hexane in water has<br>A) a large
Q8: Draw the structure of 3-hexyn-2-ol.
Q9: Name the compound. Include the appropriate stereochemical
Q10: Consider the equilibria in CCl<sub>4</sub>, an apolar,
Q11: Draw all the alcohols having a molecular