Short Answer
In each case, identify the better solvent for dissolving the compounds. Choose between H2O (ε = 78) or benzene (ε = 2.3). (Benzene is a six-carbon cyclic hydrocarbon.)
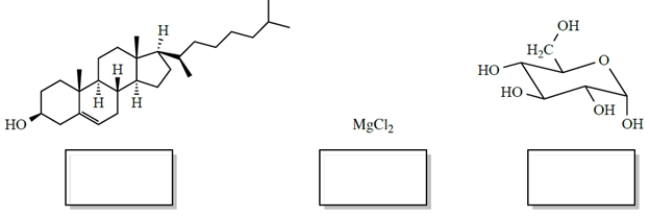
Correct Answer:

Verified
benzene; w...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
benzene; w...
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Related Questions
Q1: When two hydrocarbon molecules (such as hexane),
Q2: This is the predominant form of the
Q3: The boiling point of pentane is 36
Q5: In which solvent should NaCl (an ionic
Q6: Sodium chloride, an ionic compound, should
Q7: Dissolving hexane in water has<br>A) a large
Q8: Draw the structure of 3-hexyn-2-ol.
Q9: Name the compound. Include the appropriate stereochemical
Q10: Consider the equilibria in CCl<sub>4</sub>, an apolar,
Q11: Draw all the alcohols having a molecular