Essay
Consider this Brønsted acid-base equilibrium:
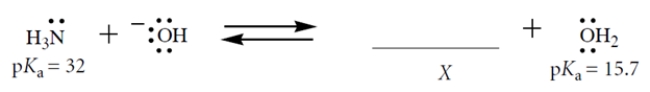 1. Fill in the structure of X; show formal charge and all valence electrons.
1. Fill in the structure of X; show formal charge and all valence electrons.
2. What is Keq for this reaction for the left-to-right direction?
3. If the reaction starts out with 1 M NaOH and 0.5 M NH3 in the solvent water (55.5 M), which species (other than water) is present in the highest concentration at equilibrium?
Correct Answer:

Verified
1. The structure of X is the conjugate b...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Select the correct statement about this
Q16: Draw curved arrows and give the products
Q17: Select the equilibrium that lies farthest to
Q18: Pentothal is a barbiturate used (as its
Q19: Pivalic acid, which has the structure (CH<sub>3</sub>)<sub>3</sub>C-CO<sub>2</sub>H,
Q20: In the reaction, CH<sub>3</sub>O<sup>−</sup> acts as a
Q22: Complete the reaction and draw the curved-arrow
Q23: Equal amount of compounds A and B
Q24: Determine the standard free-energy change of a
Q25: Predict the approximate equilibrium constant (as