Essay
In the reaction, CH3O− acts as a nucleophile, the phosphorus as an electrophilic center, and CH3S as a leaving group. (Don't be concerned that phosphorus has more than an octet of electrons-that's allowed, and it's irrelevant to the problem.)
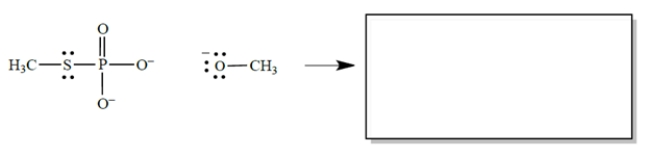 a. In the equation, draw the curved arrows for the process described.
a. In the equation, draw the curved arrows for the process described.
b. In the box, draw the products of the reaction. Don't forget formal charges.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Select the correct statement about this
Q16: Draw curved arrows and give the products
Q17: Select the equilibrium that lies farthest to
Q18: Pentothal is a barbiturate used (as its
Q19: Pivalic acid, which has the structure (CH<sub>3</sub>)<sub>3</sub>C-CO<sub>2</sub>H,
Q21: Consider this Brønsted acid-base equilibrium:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q22: Complete the reaction and draw the curved-arrow
Q23: Equal amount of compounds A and B
Q24: Determine the standard free-energy change of a
Q25: Predict the approximate equilibrium constant (as