Multiple Choice
Pentothal is a barbiturate used (as its sodium salt) as a short-acting anesthetic. Pentothal is a weak acid with a dissociation constant Ka = 4 × 10−8 M. Which one of the statements best describes the dissociation state of pentothal in the blood, which has a pH of 7.4?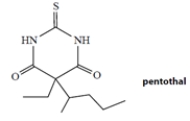
A) more than 90% dissociated
B) between 60% and 90% dissociated
C) about half dissociated
D) between 10% and 40% dissociated
E) less than 10% dissociated
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Consider the Brønsted acid-base reaction:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q14: In this acid-base equilibrium, the pK<sub>a</sub>
Q15: Select the correct statement about this
Q16: Draw curved arrows and give the products
Q17: Select the equilibrium that lies farthest to
Q19: Pivalic acid, which has the structure (CH<sub>3</sub>)<sub>3</sub>C-CO<sub>2</sub>H,
Q20: In the reaction, CH<sub>3</sub>O<sup>−</sup> acts as a
Q21: Consider this Brønsted acid-base equilibrium:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg"
Q22: Complete the reaction and draw the curved-arrow
Q23: Equal amount of compounds A and B