Essay
The Ritter reaction is a useful method for producing substituted amides. Provide a detailed, arrow-pushing mechanism for the transformation. Show all reactive intermediates and all proton transfer steps. (It is not necessary to show every resonance structure for intermediates.)
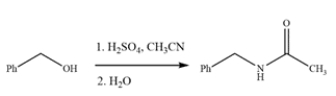
Correct Answer:

Verified
The nitrogen in the product must come fr...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q3: A student is asked to monitor the
Q4: Which reagents could be used to synthesize
Q5: Given the synthetic sequence, predict the structures
Q6: Predict the major organic product of the
Q7: When these two amides undergo hydrolysis under
Q9: Provide a detailed, arrow-pushing mechanism for the
Q10: Draw a triglyceride formed from glycerol and
Q11: Predict the major organic product of the
Q12: Predict the major organic product of the
Q13: An unlabeled flask contains a compound with