Essay
A student is asked to monitor the reaction by infrared spectroscopy. Explain what peaks the student should use to monitor the reaction (appearance and disappearance).
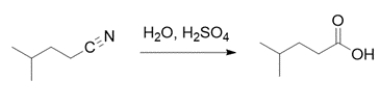
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Predict the major organic product of the
Q2: Outline a synthesis for the transformation using
Q4: Which reagents could be used to synthesize
Q5: Given the synthetic sequence, predict the structures
Q6: Predict the major organic product of the
Q7: When these two amides undergo hydrolysis under
Q8: The Ritter reaction is a useful method
Q9: Provide a detailed, arrow-pushing mechanism for the
Q10: Draw a triglyceride formed from glycerol and
Q11: Predict the major organic product of the