Essay
When these two amides undergo hydrolysis under identical reaction conditions, it is found that amide A reacts significantly faster (>1000 times) than does amide B.
a. Draw the structure of each hydrolysis product in the appropriate box.
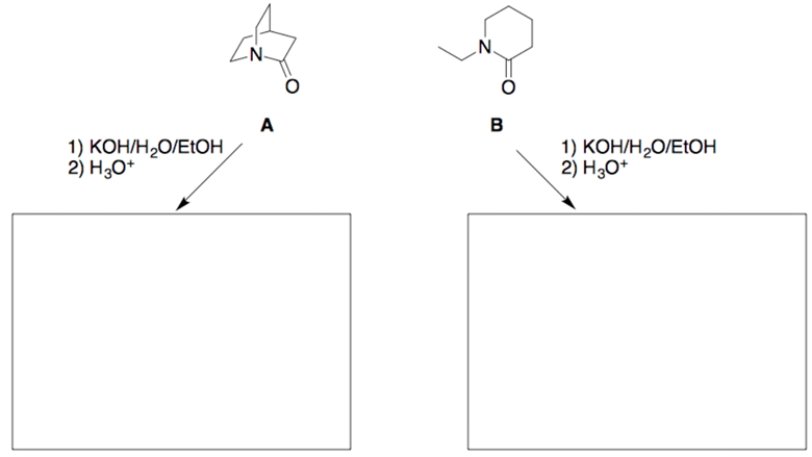 b. Using concepts developed in this course, and using structures and reaction coordinate diagrams, explain the above indicated difference in the hydrolysis rates. (Assume the ring strains in amides A and B are essentially equal, and the tetrahedral intermediates (TIs) for both reactions are of about equal energy.)
b. Using concepts developed in this course, and using structures and reaction coordinate diagrams, explain the above indicated difference in the hydrolysis rates. (Assume the ring strains in amides A and B are essentially equal, and the tetrahedral intermediates (TIs) for both reactions are of about equal energy.)
Correct Answer:

Verified
a. Each reaction is going to hydrolyze t...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q2: Outline a synthesis for the transformation using
Q3: A student is asked to monitor the
Q4: Which reagents could be used to synthesize
Q5: Given the synthetic sequence, predict the structures
Q6: Predict the major organic product of the
Q8: The Ritter reaction is a useful method
Q9: Provide a detailed, arrow-pushing mechanism for the
Q10: Draw a triglyceride formed from glycerol and
Q11: Predict the major organic product of the
Q12: Predict the major organic product of the