Essay
1,3-Butadiene, the simplest conjugated diene, has a UV absorption at 217 nm. (One nanometer = 10-9 meter.)
a. Calculate the energy of UV radiation with this wavelength (in kJ mol-1). Show your work.
b. Shown is an MO electron-occupancy diagram for the molecular orbitals of 1,3-butadiene. Draw an arrow on this diagram that corresponds to the energy of this UV radiation.
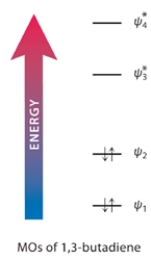
Correct Answer:

Verified
a. The relationship between the energy o...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: Determine whether each compound is aromatic, antiaromatic,
Q16: Draw a Frost circle and determine the
Q17: 1,3-Cyclopentadiene is unusually acidic, with a pK<sub>a</sub>
Q18: Select all structures that contain conjugated pi
Q19: Pyridine and pyrrole are both aromatic nitrogen-containing
Q20: The Diels-Alder reaction can give two constitutional
Q21: Which compound or ion has five <font
Q22: Cyclooctatetraene (COT) has eight pi electrons but
Q23: Which compound should have the greatest <font
Q25: Which compound has the smaller (less positive