Essay
Menthol is obtained from oils of peppermint and spearmint and is often added to cough drops to give a minty, cooling sensation. In the electron ionization mass spectrum of menthol, the molecular ion peak is not observed, but the largest fragment has an m/z 138 at 27% relative abundance and an ion at m/z 139 with a 3% relative abundance. Given the two possible compounds, identify which is menthol and explain how you arrived at that conclusion.
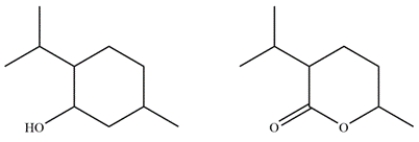
Correct Answer:

Verified
Both compounds have a molecular weight o...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q15: The electron ionization mass spectrum of 1-hexanol
Q16: The IR absorption that occurs at the
Q17: These two compounds have similar molecular weights.
Q18: The electron ionization of an unknown neutral
Q19: A graduate student is cleaning out the
Q20: Typical IR absorptions occur in the 650-3700
Q21: When the epoxy thiol is treated with
Q22: The base peak of 2,2-dimethylpentane is at
Q24: Select the true statement.<br>A) The S-H stretching
Q25: Which of the compounds would have the