Essay
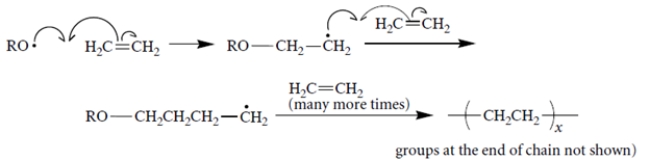
Free-radical polymerization of ethylene proceeds with the following propagation steps.
 It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
It turns out that polyethylene made by free-radical polymerization contains some branches, for example,
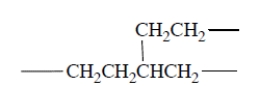 (a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(a) Give a plausible mechanism, complete with "fishhooks," for propagation steps that can account for formation of these branches.
(b) It is possible (by a completely different method) to form polyethylene with unbranched chains. Which do you think would have higher density, branched-chain polyethylene or polyethylene with unbranched chains? Explain briefly.
Correct Answer:

Verified
(a) A radical abstracts a hydrogen atom ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Thiols (R-S-H) undergo addition to alkenes
Q2: Complete the reactions by giving the structure
Q3: Identify the stereochemistry of the polymers.<br> <img
Q4: Outline a synthesis of this product.<br> <img
Q6: Devise a synthesis to achieve the transformation.<br>
Q7: Give the structure of the organic free-radical
Q8: Give the product A that results when
Q9: 1-Methyl-1-vinyl cyclopentane undergoes addition of HBr under
Q10: A free-radical addition of a certain
Q11: Complete the reaction by giving the major