Multiple Choice
Thiols (R-S-H) undergo addition to alkenes but only in the presence of peroxides. This equation is an example.
Which one of these is a reactive intermediate in this reaction?
(a)
(b) 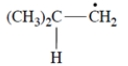
(c)
(d)
(e) 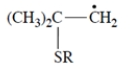
(f)
A) compound (a)
B) compound (b)
C) compound (c)
D) compound (d)
E) compound (e) f.
compound (f)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Complete the reactions by giving the structure
Q3: Identify the stereochemistry of the polymers.<br> <img
Q4: Outline a synthesis of this product.<br> <img
Q5: Free-radical polymerization of ethylene proceeds with the
Q6: Devise a synthesis to achieve the transformation.<br>
Q7: Give the structure of the organic free-radical
Q8: Give the product A that results when
Q9: 1-Methyl-1-vinyl cyclopentane undergoes addition of HBr under
Q10: A free-radical addition of a certain
Q11: Complete the reaction by giving the major