Essay
Complete the electron configuration diagram below for the element boron (B, atomic number = 5), showing 1s, 2s, 2px, 2py, and 2pz orbitals, their relative energies, and their electron populations indicated by "spin arrows" ↑ and ↓.
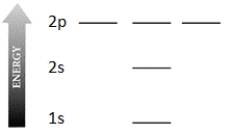
Correct Answer:

Verified
Correct Answer:
Verified
Q10: According to molecular orbital theory, which
Q11: The atomic orbital that cannot exist is<br>A)
Q12: How many valence electrons does aluminum (Al,
Q13: Identify the true statement(s) about molecular orbitals
Q14: Consider this structure:<br> <span class="ql-formula" data-value="\mathrm
Q16: What is the geometry of the borohydride
Q17: Complete the structure for the cyanate ion
Q18: Given that the acetate anion has the
Q19: Add valence electrons to the structures so
Q20: Which compound has the largest dipole moment?<br><img