Multiple Choice
Given that the acetate anion has the these equally important resonance structures, what is the bond order of each carbon-oxygen bond?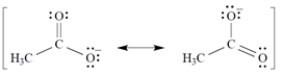
A) The bond order of all carbon-oxygen bonds is ⅓.
B) The bond order of all carbon-oxygen bonds is ½.
C) The bond order of all carbon-oxygen bonds is 1.0.
D) The bond order of all carbon-oxygen bonds is 1.5.
E) The bond order of one carbon-oxygen bond is 1.0, and the bond order of the other carbon-oxygen is ½.f.
The bond order of all carbon-oxygen bonds is 2.0.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Identify the true statement(s) about molecular orbitals
Q14: Consider this structure:<br> <span class="ql-formula" data-value="\mathrm
Q15: Complete the electron configuration diagram below for
Q16: What is the geometry of the borohydride
Q17: Complete the structure for the cyanate ion
Q19: Add valence electrons to the structures so
Q20: Which compound has the largest dipole moment?<br><img
Q21: Give the formal charge on the phosphorus
Q22: Assume that the structure of BF<sub>3</sub> (boron
Q23: Consider this compound:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider this