Essay
Add valence electrons to the structures so that the formal charge is properly accounted for. Assume that all atoms have no more electrons than allowed by the octet rule. Make your "electron dots" bold enough to be unambiguous.
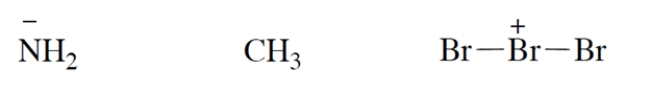
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Consider this structure:<br> <span class="ql-formula" data-value="\mathrm
Q15: Complete the electron configuration diagram below for
Q16: What is the geometry of the borohydride
Q17: Complete the structure for the cyanate ion
Q18: Given that the acetate anion has the
Q20: Which compound has the largest dipole moment?<br><img
Q21: Give the formal charge on the phosphorus
Q22: Assume that the structure of BF<sub>3</sub> (boron
Q23: Consider this compound:<br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBMC1048/.jpg" alt="Consider this
Q24: What is the C-N-O bond angle in