Multiple Choice
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 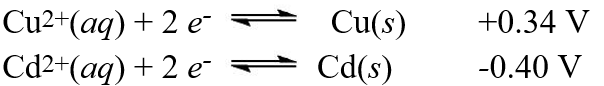 What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
A) +12 kJ
B) -12 kJ
C) +143 kJ
D) -143 kJ
E) -71 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Table of standard electrode potentials <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/.jpg"
Q21: Cations<br>A)are negatively charged ions that result from
Q22: The SI unit for electric current is
Q23: In lithium ion batteries, lithium ions are
Q24: The salt bridge in a galvanic cell
Q26: Consider these metal ion/metal standard reduction potentials
Q27: Fuel cells are different from other traditional
Q28: Using the standard reduction potentials <img
Q29: How long would it take to deposit
Q30: Table of standard electrode potentials <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/.jpg"