Multiple Choice
Table of standard electrode potentials 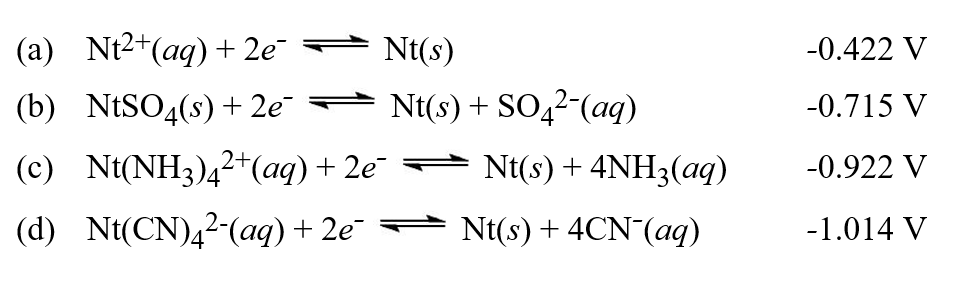
-Using a table of standard electrode potentials, decide which of the following statements is completely true.
A) Cl2 can oxidize Cd, and Al3+ can reduce Cu2+.
B) Cd2+ can oxidize Cu, and Cd2+ can reduce Cl2.
C) Cu2+ can oxidize Cd, and Cd can reduce Al3+.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: A galvanic cell is composed of
Q26: Consider these metal ion/metal standard reduction potentials
Q27: Fuel cells are different from other traditional
Q28: Using the standard reduction potentials <img
Q29: How long would it take to deposit
Q31: Why is cryolite, Na<sub>3</sub>AlF<sub>6</sub>, mixed with aluminum
Q32: A galvanic cell consists of a Cu(s)|Cu<sup>2+</sup>(aq)half-cell
Q33: Lithium metal can be produced readily by
Q34: Using these metal ion/metal standard reduction
Q35: In doping semiconductor materials,<br>A)the energy gap between