Multiple Choice
Using the standard reduction potentials 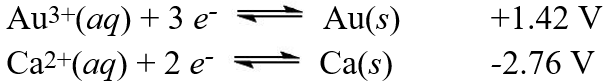 Calculate the standard free energy ( G°) change for the cell reaction:
Calculate the standard free energy ( G°) change for the cell reaction:
2 Au(s) + 3 Ca2+(aq) 2 Au3+(aq) + 3 Ca(s)
A) 2420 kJ
B) 388 kJ
C) -766 kJ
D) 766 kJ
E) -1210 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q23: In lithium ion batteries, lithium ions are
Q24: The salt bridge in a galvanic cell
Q25: A galvanic cell is composed of
Q26: Consider these metal ion/metal standard reduction potentials
Q27: Fuel cells are different from other traditional
Q29: How long would it take to deposit
Q30: Table of standard electrode potentials <img src="https://d2lvgg3v3hfg70.cloudfront.net/TBW1039/.jpg"
Q31: Why is cryolite, Na<sub>3</sub>AlF<sub>6</sub>, mixed with aluminum
Q32: A galvanic cell consists of a Cu(s)|Cu<sup>2+</sup>(aq)half-cell
Q33: Lithium metal can be produced readily by