Multiple Choice
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 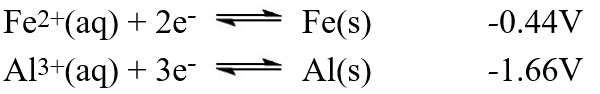 What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
A) -806 kJ
B) -1.22 × 103 kJ
C) -706 kJ
D) -540 kJ
E) -600 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q32: A galvanic cell consists of a Cu(s)|Cu<sup>2+</sup>(aq)half-cell
Q33: Lithium metal can be produced readily by
Q34: Using these metal ion/metal standard reduction
Q35: In doping semiconductor materials,<br>A)the energy gap between
Q36: The cathode in a galvanic cell has
Q38: Using the same current and similar conditions,
Q39: Using the standard reduction potentials <img
Q40: A galvanic cell is composed of these
Q41: Using the standard reduction potentials: <img
Q42: The cell described by the reaction,2 Co<sup>3+</sup>(aq)+