Multiple Choice
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 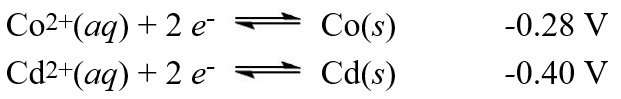 What is the standard free energy change for the cell reaction of this galvanic cell?
What is the standard free energy change for the cell reaction of this galvanic cell?
A) -12 kJ
B) +12 kJ
C) -23 kJ
D) +23 kJ
E) -46 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q35: In doping semiconductor materials,<br>A)the energy gap between
Q36: The cathode in a galvanic cell has
Q37: A galvanic cell is composed of
Q38: Using the same current and similar conditions,
Q39: Using the standard reduction potentials <img
Q41: Using the standard reduction potentials: <img
Q42: The cell described by the reaction,2 Co<sup>3+</sup>(aq)+
Q43: When an aqueous solution of AgNO<sub>3</sub> is
Q44: A galvanic cell is composed of
Q45: For the reaction, 2 Cr<sup>2+</sup>(aq)+ Cl<sub>2</sub>(g)