Multiple Choice
Using the standard reduction potentials 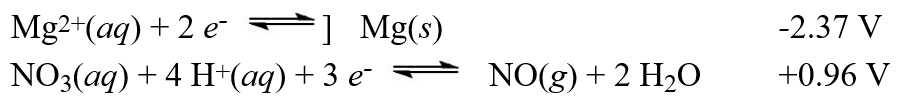 Calculate the value of E°cell for the cell with the reaction:
Calculate the value of E°cell for the cell with the reaction:
3 Mg(s) + 2 NO3-(aq) + 8 H+(aq) 3 Mg2+(aq) + 2 NO(g) + 4 H2O
A) +1.41 V
B) -1.41 V
C) +3.33 V
D) +8.46 V
E) -8.46 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q34: Using these metal ion/metal standard reduction
Q35: In doping semiconductor materials,<br>A)the energy gap between
Q36: The cathode in a galvanic cell has
Q37: A galvanic cell is composed of
Q38: Using the same current and similar conditions,
Q40: A galvanic cell is composed of these
Q41: Using the standard reduction potentials: <img
Q42: The cell described by the reaction,2 Co<sup>3+</sup>(aq)+
Q43: When an aqueous solution of AgNO<sub>3</sub> is
Q44: A galvanic cell is composed of