Multiple Choice
A galvanic cell is composed of these two half-cells, with the standard reduction potentials shown: 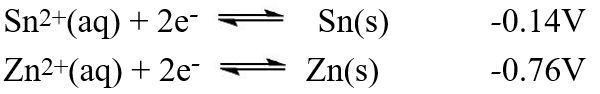 What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
What is the standard free energy ( G°) change for the cell reaction of this galvanic cell?
A) -2.22 × 102 kJ
B) -3.14 × 102 kJ
C) -1.74 × 102 kJ
D) -6.02 × 102 kJ
E) -1.20 × 102 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Using the standard reduction potentials <img
Q40: A galvanic cell is composed of these
Q41: Using the standard reduction potentials: <img
Q42: The cell described by the reaction,2 Co<sup>3+</sup>(aq)+
Q43: When an aqueous solution of AgNO<sub>3</sub> is
Q45: For the reaction, 2 Cr<sup>2+</sup>(aq)+ Cl<sub>2</sub>(g)
Q46: When an aqueous solution of sodium sulfate
Q47: Consider these metal ion/metal standard reduction potentials
Q48: In the zinc-carbon dry cell, the electrode
Q49: Steel objects that are exposed to weather