Short Answer
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 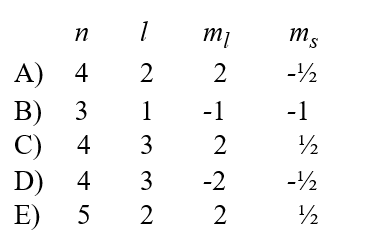
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q158: Which statement is true of Bohr's equation,
Q159: The increase in atomic radius as one
Q160: The directions of maximum electron density of
Q161: What is the energy of one photon
Q162: Calculate the wavelength of a particle of
Q164: Given the following sets of quantum numbers
Q165: Which of the following choices is the
Q166: The maximum number of electrons in an
Q167: Calculate the frequency of visible light having
Q168: The following are the ionization energies for