Short Answer
Given the following sets of quantum numbers for n, l, ml, and ms,which one of these sets is not possible for an electron in an atom? 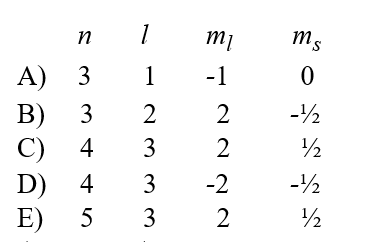
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q159: The increase in atomic radius as one
Q160: The directions of maximum electron density of
Q161: What is the energy of one photon
Q162: Calculate the wavelength of a particle of
Q163: Given the following sets of quantum numbers
Q165: Which of the following choices is the
Q166: The maximum number of electrons in an
Q167: Calculate the frequency of visible light having
Q168: The following are the ionization energies for
Q169: What is the energy of one mole