Short Answer
The maximum number of electrons in an atom that can have the following exact same set of quantum numbers is ________. 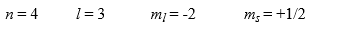
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q161: What is the energy of one photon
Q162: Calculate the wavelength of a particle of
Q163: Given the following sets of quantum numbers
Q164: Given the following sets of quantum numbers
Q165: Which of the following choices is the
Q167: Calculate the frequency of visible light having
Q168: The following are the ionization energies for
Q169: What is the energy of one mole
Q170: Calculate the wavelength of a helium atom
Q171: In the tellurium atom, the electron configuration