Essay
The following concentration vs. time data were collected for the reaction:
A + 2B C 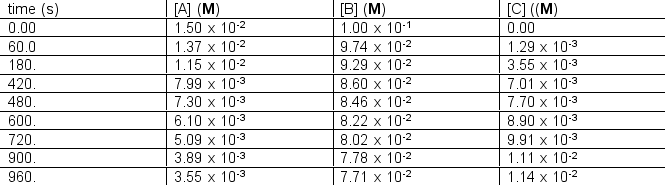 Calculate
Calculate 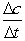 for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
for A, B and C for the following time differences:(a) 0 and 60 s,(b) 900 and 960 s,(c) What is the rate of the reaction for part
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: For the following reaction A +
Q61: What are the units of a rate
Q62: Write the overall equation of reaction
Q63: Explain the mechanisms by which catalysts function.
Q64: Consider the following energy-reaction coordinate diagram. <img
Q66: Write the overall equation of reaction
Q67: Consider the following three molecular pictures
Q68: Rate data were collected for the
Q69: The following mechanism has been suggested
Q70: The synthesis of nitrogen monoxide proceeds