Essay
Consider the following three molecular pictures that represent the relative numbers of the two reactants involved in one step of the depletion of stratospheric ozone by chlorine atoms: 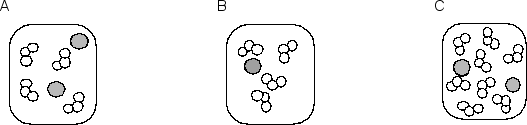 The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2
The equation for the elementary reaction and a molecular picture of the reaction process are shown below:Cl• + O3 ClO + O2 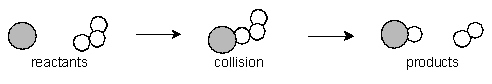 If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
If the three samples represented by A, B and C are at the same temperature, what are the rates of reaction of B and C compared to that of A?
Correct Answer:

Verified
In B the reaction is...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q62: Write the overall equation of reaction
Q63: Explain the mechanisms by which catalysts function.
Q64: Consider the following energy-reaction coordinate diagram. <img
Q65: The following concentration vs. time data
Q66: Write the overall equation of reaction
Q68: Rate data were collected for the
Q69: The following mechanism has been suggested
Q70: The synthesis of nitrogen monoxide proceeds
Q71: What is the rate law associated
Q72: The first-order rate constant for the decomposition