Short Answer
Rate data were collected for the following reaction at a constant temperature.2ClO2(aq) + 2 OH-1(aq) ClO3-1(aq) + ClO2-1(aq) + H2O(l) 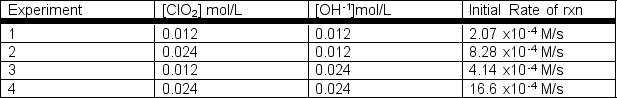 a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
a) Determine the rate law for this reaction.b) Determine the rate constant with appropriate units.
Correct Answer:

Verified
a) rate = k[ClO...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q63: Explain the mechanisms by which catalysts function.
Q64: Consider the following energy-reaction coordinate diagram. <img
Q65: The following concentration vs. time data
Q66: Write the overall equation of reaction
Q67: Consider the following three molecular pictures
Q69: The following mechanism has been suggested
Q70: The synthesis of nitrogen monoxide proceeds
Q71: What is the rate law associated
Q72: The first-order rate constant for the decomposition
Q73: The activation energy for the high temperature