Short Answer
Ammonium cyanate undergoes rearrangement to form urea in aqueous solution.NH4NCO(aq) CO(NH2)2 (aq)The following data was collected: 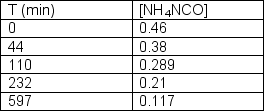 Determine the order of the reaction and the rate constant.
Determine the order of the reaction and the rate constant.
Correct Answer:

Verified
2ndView Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q29: The rate law for the reaction2
Q30: Which of the following sketches shows the
Q31: The formation of chlorocarbon solvents such
Q32: The reaction of nitrogen dioxide and
Q33: Radioactive decay follows first-order kinetics. Some smoke
Q35: For the reaction I<sup>-</sup>(aq) + OCl<sup>-</sup>(aq)
Q36: Which of the following is an
Q37: Nitrous oxide, N<sub>2</sub>O, decomposes on metal
Q38: The following are initial rate data for
Q39: The three reactions below, with identical