The Reaction of Nitrogen Dioxide and Fluorine Is:2 NO2 2 NO2FOne Proposed Mechanism Has Two Steps; the First Step
Short Answer
The reaction of nitrogen dioxide and fluorine is:2 NO2 + F2 2 NO2FOne proposed mechanism has two steps; the first step is rate determining: 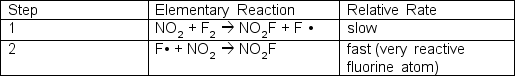 What is the experimentally determined rate law?
What is the experimentally determined rate law?
Correct Answer:

Verified
rate = kobsView Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q27: Determine the rate of a reaction based
Q28: Cyclohexane is manufactured from the reaction
Q29: The rate law for the reaction2
Q30: Which of the following sketches shows the
Q31: The formation of chlorocarbon solvents such
Q33: Radioactive decay follows first-order kinetics. Some smoke
Q34: Ammonium cyanate undergoes rearrangement to form
Q35: For the reaction I<sup>-</sup>(aq) + OCl<sup>-</sup>(aq)
Q36: Which of the following is an
Q37: Nitrous oxide, N<sub>2</sub>O, decomposes on metal