Multiple Choice

Determining Oxidation Numbers
-What is the oxidation number of the carbon in bold print in the following structure? 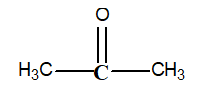
A) -1
B) +2
C) -3
D) +4
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q51: What is the oxidation state of the
Q52: Which is the strongest oxidizing agent?<br>A) Ce<sup>4+</sup><br>B)
Q53: What is the standard cell potential
Q54: Oxidation-reduction reactions are often written with double
Q55: Given the following half-cell reduction potentials, determine
Q57: What is the potential for a voltaic
Q58: The equilibrium constant at 25 <sup>o</sup>C
Q59: Write a balanced chemical equation for
Q60: Balance the following oxidation-reduction equation.<br>CrO<sub>4</sub><sup>2-</sup>(aq) + HSnO<sub>2</sub><sup>-</sup>(aq)<br>
Q61: What is the correct coefficient for