Multiple Choice
Oxidation-reduction reactions are often written with double arrows indicating that the reaction can go in either direction depending on the experimental conditions. For the following reaction identify the oxidizing agent on both sides of the chemical equation.
S2O82-(aq) + Zn(s) 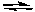 Zn2+(aq) + 2 SO42-(aq)
Zn2+(aq) + 2 SO42-(aq)
A) S2O82- and SO42-
B) Zn and SO42-
C) Zn and Zn2+
D) S2O82- and Zn2+
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q49: Consider the following generalized half-reactions.<br>A<sub>ox</sub> + e<sup>-</sup>
Q50: How many electrons are transferred in the
Q51: What is the oxidation state of the
Q52: Which is the strongest oxidizing agent?<br>A) Ce<sup>4+</sup><br>B)
Q53: What is the standard cell potential
Q55: Given the following half-cell reduction potentials, determine
Q56: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q57: What is the potential for a voltaic
Q58: The equilibrium constant at 25 <sup>o</sup>C
Q59: Write a balanced chemical equation for