Multiple Choice
Given the following half-cell reduction potentials, determine which of the following is the strongest reducing agent.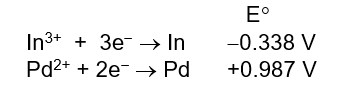
A) In3+
B) In
C) Pd2+
D) Pd
E) Cannot be determined from cell potentials alone
Correct Answer:

Verified
Correct Answer:
Verified
Q50: How many electrons are transferred in the
Q51: What is the oxidation state of the
Q52: Which is the strongest oxidizing agent?<br>A) Ce<sup>4+</sup><br>B)
Q53: What is the standard cell potential
Q54: Oxidation-reduction reactions are often written with double
Q56: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q57: What is the potential for a voltaic
Q58: The equilibrium constant at 25 <sup>o</sup>C
Q59: Write a balanced chemical equation for
Q60: Balance the following oxidation-reduction equation.<br>CrO<sub>4</sub><sup>2-</sup>(aq) + HSnO<sub>2</sub><sup>-</sup>(aq)<br>