Multiple Choice
How many electrons are transferred in the following oxidation-reduction reaction?
3 Sn2+(aq) + Cr2O72-(aq) + 14 H+ 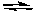 3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
3 Sn4+(aq) + 2 Cr3+(aq) + 7 H2O(l)
A) 2
B) 6
C) 12
D) 14
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q45: Given the following half-cell reduction potentials,
Q46: The equilibrium constant at 298 K
Q47: Given the following redox reactions and
Q48: The carbon in bold in the ethanol
Q49: Consider the following generalized half-reactions.<br>A<sub>ox</sub> + e<sup>-</sup>
Q51: What is the oxidation state of the
Q52: Which is the strongest oxidizing agent?<br>A) Ce<sup>4+</sup><br>B)
Q53: What is the standard cell potential
Q54: Oxidation-reduction reactions are often written with double
Q55: Given the following half-cell reduction potentials, determine