Multiple Choice
Which of the following is the best Lewis structure for carbonic acid (H2CO3) ?
A) 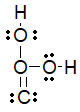
B) 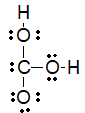
C) 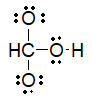
D) 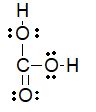
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: What can we conclude from the fact
Q61: What is the conjugate acid of hydrogen
Q62: When would the pH of a solution
Q63: What is the pH of a solution
Q64: Which of the following would be the
Q66: A diprotic acid, H<sub>2</sub>A, has the following
Q67: Which of the following acids would have
Q68: The salt NaF is dissolved in water.
Q69: Explain the following observation: Water becomes acidic
Q70: For a weak diprotic acid, H<sub>2</sub>A, for