Multiple Choice
What can we conclude from the fact that the following reaction proceeds as written?
NaNH2(s) + H2O(l) 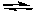 NH3(aq) + OH-(aq)
NH3(aq) + OH-(aq)
A) OH- is a stronger base than NH2-.
B) NH3 is a stronger acid than H2O.
C) NH2- is a stronger acid than H2O.
D) OH- is a weaker base than NH2-.
E) More than one of the above are true.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: Predict the products of the following acid-base
Q56: Which of the following solutions has a
Q57: What is the pH of a 0.0250
Q58: Which of the following solutions has the
Q59: Which of the following compounds is not
Q61: What is the conjugate acid of hydrogen
Q62: When would the pH of a solution
Q63: What is the pH of a solution
Q64: Which of the following would be the
Q65: Which of the following is the best