Multiple Choice
What is the pH of a solution that is 0.01 M in CH3NH2 and 0.1 M in CH3NH3+?
CH3NH2(aq) + H2O(l) 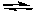 CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
A) 2.36
B) 3.36
C) 9.64
D) 10.64
E) 11.64
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q58: Which of the following solutions has the
Q59: Which of the following compounds is not
Q60: What can we conclude from the fact
Q61: What is the conjugate acid of hydrogen
Q62: When would the pH of a solution
Q64: Which of the following would be the
Q65: Which of the following is the best
Q66: A diprotic acid, H<sub>2</sub>A, has the following
Q67: Which of the following acids would have
Q68: The salt NaF is dissolved in water.