Multiple Choice
Assume that the equilibrium constants for the following reactions are known.
2 SO2(g) + O2(g) 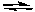 2 SO3(g) K1
2 SO3(g) K1
2 CO(g) + O2(g) 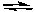 2 CO2(g) K2
2 CO2(g) K2
What is the equilibrium constant for the following reaction?
2 SO2(g) + 2 CO2(g) 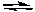 2 SO3(g) + 2 CO(g)
2 SO3(g) + 2 CO(g)
A) K = K1 x K2
B) K = K1/K2
C) K = K2/K1
D) K = K1 + K2
E) K = K1 - K2
Correct Answer:

Verified
Correct Answer:
Verified
Q62: Which of the following initial concentrations of
Q63: At equilibrium, _.<br>A) all chemical processes
Q64: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q65: A solution is prepared in which the
Q66: What would happen to the extent of
Q68: A 0.100 M sample of SO<sub>2</sub> reacts
Q69: What is the concentration of Ag<sup>+</sup> (mol/L)
Q70: Which of the following equations describes
Q71: Calculate the concentrations of all reactants and
Q72: The following reactions are at equilibrium. Beside