Multiple Choice
Which of the following initial concentrations of reactants and products will cause the reaction to proceed to the right in order to establish equilibrium?
2 NO2(g) 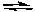 2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200
2 NO(g) + O2(g) Kc=3.4*10 -F 7 (at 200 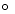 C)
C) 
A) 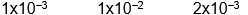
B) 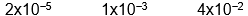
C) 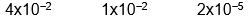
D) 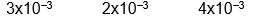
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q57: The following reaction was carried out:<br>Ni(CO)<sub>4</sub>
Q58: Which of the following equations describes
Q59: What is the solubility in moles per
Q60: Describe the relationship between the rate constants
Q61: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q63: At equilibrium, _.<br>A) all chemical processes
Q64: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q65: A solution is prepared in which the
Q66: What would happen to the extent of
Q67: Assume that the equilibrium constants for the