Multiple Choice
What would happen to the extent of the decomposition of PCl5 at a given temperature if the pressure on the system were decreased by increasing the volume of the container at a constant temperature?
PCl5(g) 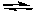 PCl3(g) + Cl2(g)
PCl3(g) + Cl2(g)
A) It would increase.
B) It would decrease.
C) It would remain constant.
D) Not enough information is given to determine this.
Correct Answer:

Verified
Correct Answer:
Verified
Q61: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q62: Which of the following initial concentrations of
Q63: At equilibrium, _.<br>A) all chemical processes
Q64: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q65: A solution is prepared in which the
Q67: Assume that the equilibrium constants for the
Q68: A 0.100 M sample of SO<sub>2</sub> reacts
Q69: What is the concentration of Ag<sup>+</sup> (mol/L)
Q70: Which of the following equations describes
Q71: Calculate the concentrations of all reactants and