Multiple Choice
The following reaction was carried out at 250C with the initial concentration of NO2 being 0.70 M and no NO(g) or O2(g) initially present. At equilibrium the NO2 concentration was found to be 0.28 M. Calculate Kc for the reaction.
2NO2(g) 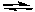 2NO(g) + O2(g)
2NO(g) + O2(g)
A) 0.47
B) 0.94
C) 1.9
D) 0.14
E) 2.1
Correct Answer:

Verified
Correct Answer:
Verified
Q78: For the reaction shown below, at equilibrium<br>I<sub>2</sub>(g)
Q79: What is the effect of increasing the
Q80: The following reactions are at equilibrium. Which
Q81: What is the K<sub>sp</sub> of SrF<sub>2</sub>(s) if
Q82: The following reaction is at equilibrium. Which
Q84: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q85: Use the following graph for answering <br>
Q86: If 1.3 x 10 <sup>-</sup> <sup>5</sup> moles
Q87: For the reaction below, 0.0500 moles of
Q88: The following chemical reaction has reached