Multiple Choice
The following reaction is at equilibrium. Which of the conditions listed below will cause the reaction to shift toward the products?
2 SO3(g) + 2 Cl2(g) 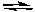 2 SO2Cl2(g) + O2(g)
2 SO2Cl2(g) + O2(g)
A) The concentration of O2 is increased.
B) The concentration of SO2Cl2 is decreased.
C) The concentration of SO3 is decreased.
D) The pressure is decreased.
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q77: What is the correct solubility constant expression
Q78: For the reaction shown below, at equilibrium<br>I<sub>2</sub>(g)
Q79: What is the effect of increasing the
Q80: The following reactions are at equilibrium. Which
Q81: What is the K<sub>sp</sub> of SrF<sub>2</sub>(s) if
Q83: The following reaction was carried out at
Q84: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q85: Use the following graph for answering <br>
Q86: If 1.3 x 10 <sup>-</sup> <sup>5</sup> moles
Q87: For the reaction below, 0.0500 moles of