Essay
Calculate the NO, NO2, and O2 concentrations in the following gas-phase reaction at equilibrium at 200°C.
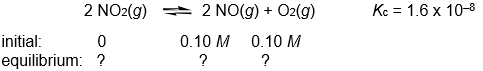
Correct Answer:

Verified
[NO] = 5.6...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q2: For the following reaction 2 NO<sub>2</sub>(g) <img
Q3: What would be the effect of removing
Q5: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q6: What is the correct solubility constant expression
Q7: What is the concentration in moles per
Q8: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q9: Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub>
Q10: In which of the following will the
Q11: If the equilibrium constant for the following