Multiple Choice
If the equilibrium constant for the following reaction is Kc = 1 x 102
2 C2H6(g) + 7 O2(g) 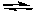 4 CO2(g) + 6 H2O(g)
4 CO2(g) + 6 H2O(g)
And all the concentrations were initially 0.10 M, we can predict that the reaction
A) is at equilibrium initially.
B) must shift from left to right to reach equilibrium.
C) must shift from right to left to reach equilibrium.
D) cannot reach equilibrium.
E) cannot be determined unless we have the information necessary to calculate Qc for the reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q6: What is the correct solubility constant expression
Q7: What is the concentration in moles per
Q8: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q9: Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub>
Q10: In which of the following will the
Q12: Under which set of conditions must the
Q13: Which is the correct equilibrium constant expression
Q14: Hidden Assumptions that make Equilibrium Calculations Easier
Q15: Calculate the COCl<sub>2</sub>, CO, and Cl<sub>2</sub> concentrations
Q16: Which of the following factors will